A recall has been issued on a brand of extended-release capsules that may have incorrect dosage in a specific lot of bottles.
According to Health Canada, the recall has been issued by Ethypharm Inc., the manufacturer of M-Eslon morphine sulfate tablets.
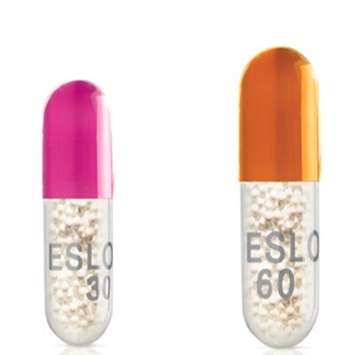
Some bottles that were labeled as 30-milligram capsules may contain 60-milligram capsules. These could result in potential overdoses that may cause serious health issues.
"Taking too much morphine or suddenly increasing the dose could potentially lead to an overdose, which can be life-threatening," read the recall notice. "Symptoms of morphine overdose may include confusion, dizziness, drowsiness, difficulties waking up, small [pinpoint] pupils, slow heartbeat, slow and difficult breathing, cold and clammy skin, fainting, seizure, coma, and death."
The 60-milligram capsule has an orange opaque cap and a clear bottom, while the 30-milligram capsule has an opaque pink cap and a clear bottom.
The lot number is 43885, with an expiry date of April 2026.
If you have a prescription for M-Eslon, check your capsules carefully. If you are prescribed the 30-milligram capsules and there are 60-milligram ones in the bottle, do not take them and call your pharmacy immediately. The pharmacist will check the prescription and provide a replacement if needed.
If you or someone you are with is experiencing an overdose, call 911 immediately.